Barth Syndrome Treatment Market Size and Trends
The Global Barth Syndrome Treatment Market is estimated to be valued at USD 160.02 billion in 2025 and is expected to reach USD 386.13 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 13.4% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The market for Barth Syndrome treatment has been witnessing prominent growth over the past few years and this trend is expected to continue in the future as well. Factors such as increasing research and development activities related to orphan drug development, rising prevalence of the rare disease Barth Syndrome worldwide, and growing awareness about treatment options are anticipated to drive the demand for Barth Syndrome treatment during the forecast period. Various drug makers are continuously focusing on developing highly pricey orphan drugs for treating rare diseases like Barth Syndrome, which is further augmenting the market expansion. However, stringent regulations for orphan drug approval and high costs associated with drug development may hamper the growth of the Barth Syndrome treatment market.
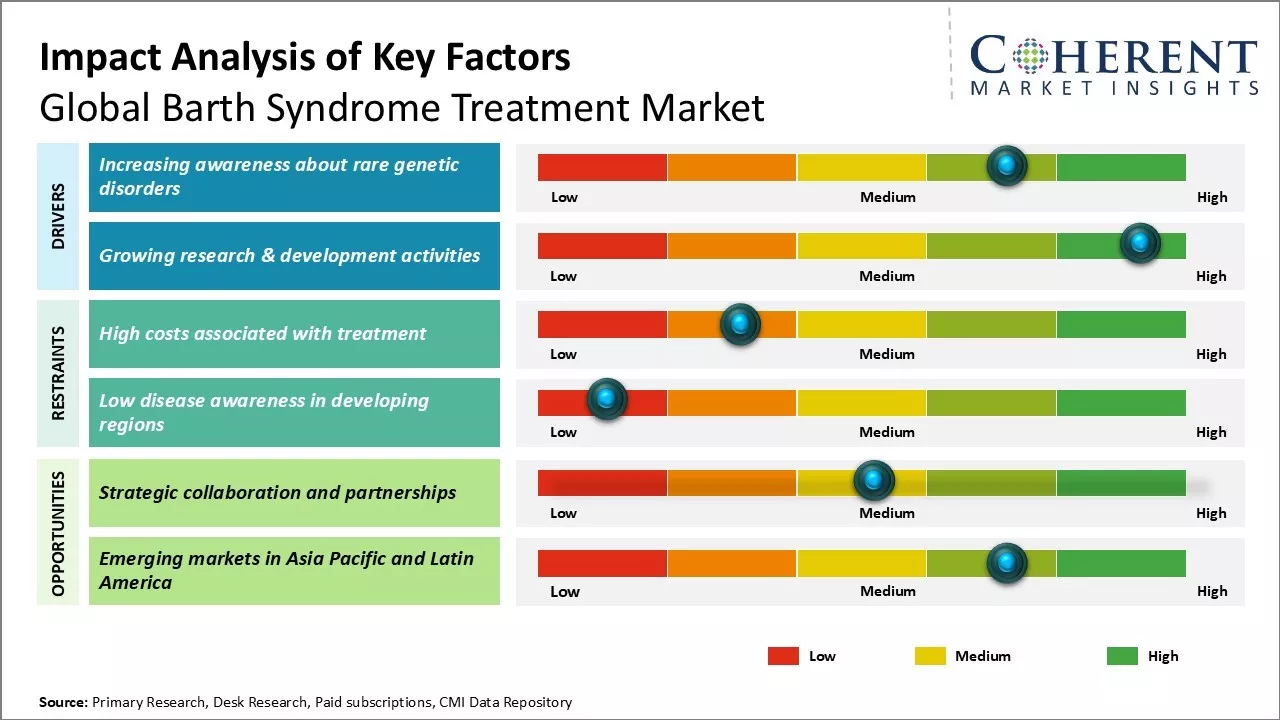
Market Driver - Increasing awareness about rare genetic disorders
One of the primary drivers fueling growth of the Barth syndrome treatment market is the rising awareness about rare genetic disorders globally. Barth syndrome is one such rare genetic disorder affecting males primarily and is characterized by cardiac abnormalities, skeletal muscle weakness and neutropenia. For many years, this condition remained highly unknown and under-diagnosed worldwide due to its rarity. However, over the past decade, dedicated patient advocacy and support groups have undertaken various initiatives to spread information about this disorder and its symptoms among healthcare professionals and the general public alike.
As awareness streams in, governments, and NGOs are also showing commitment towards rare disease treatments. Support in terms of research funding, healthcare policy formulation, newborn screening programs, and availability of care facilities are expanding coverage. All of these awareness generation and supportive initiatives are working together to bring more Barth syndrome cases to light. This will augment the patient base seeking clinical management of symptoms through available drugs and other therapies, subsequently driving higher demand for Barth syndrome treatment over the coming years.
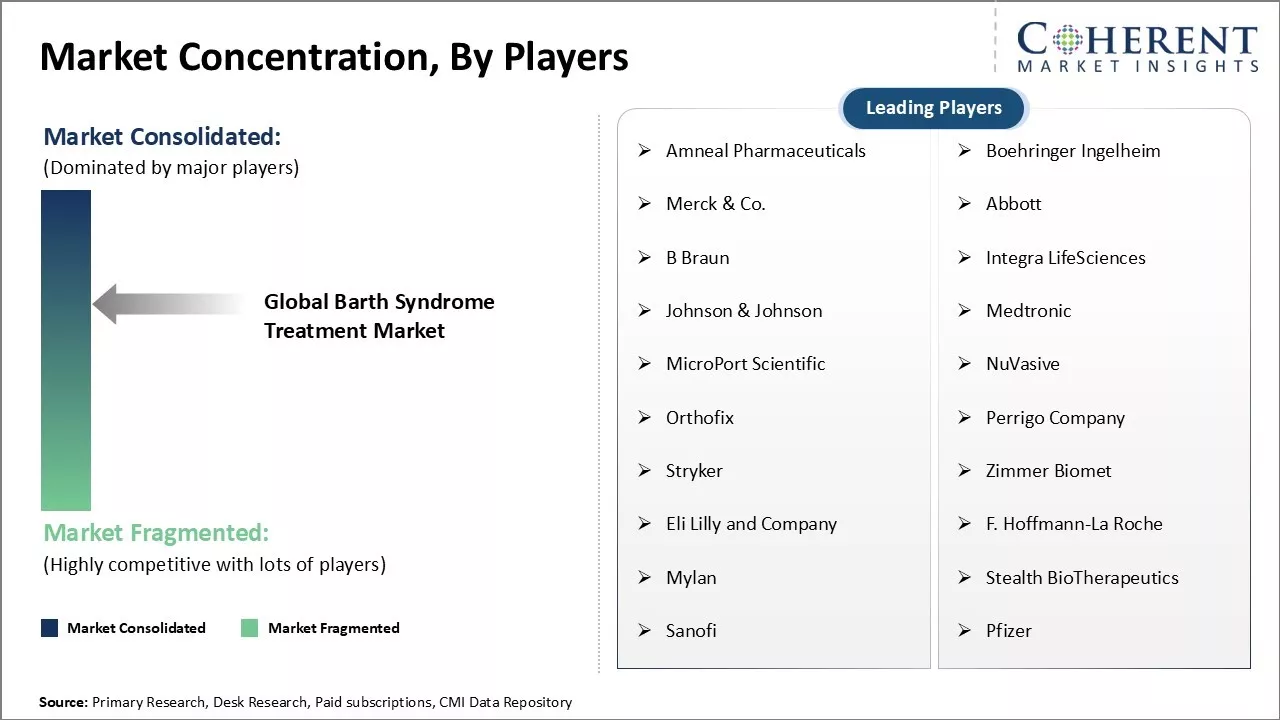
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Growing Research & Development Activities
With increasing understanding about the condition prevalence, pathogenesis, and clinical impact on patients, investment towards R&D for more effective Barth syndrome treatment is substantially rising. Multiple biopharmaceutical companies and research institutes across the globe have intensified their research efforts targeted towards developing new therapeutic drugs and cell or gene-based therapies.
A number of clinical trials are currently ongoing to evaluate drug candidates having fibroblast fatty acid uptake inhibitory activities or cardiac myocyte cell therapies for improving outcomes in Barth syndrome patients. Some studies are also exploring the applications of gene therapies involving antisense oligonucleotides or rAAV vectors to correct the genetic mutations causing this disorder. Partnerships between industry players and research organizations are further expediting the discovery of novel biomarkers and drug targets.
Venture capital funding available for orphan drug development has surged considerably over the last 5 years. This is providing necessary capital to transform research leads into actual treatment options for patients. Regulatory policies are also evolving to support expedited review paths for potential drugs addressing rare disease conditions having no current remedies. The orphan drug designation status by regulatory agencies facilitates exclusive market rights post approval and market authorization for clinical use. All these activities in R&D are anticipated to result into new product commercialization gradually, thereby accelerating Barth syndrome treatment market growth.
Key Takeaways from Analyst:
The global Barth syndrome treatment market is expected to grow significantly over the coming years. This rare genetic disorder primarily affecting boys primarily driven by increasing awareness about the disease and improving diagnosis rate worldwide. North America currently dominates the market due to high healthcare expenditure and growing R&D activities for orphan drugs in the region. However, Asia Pacific region is likely to showcase fastest market growth during the forecast period attributed to rise in healthcare facilities and spending in major countries like China and India.
The market offers ample opportunities for manufacturers as there remains high unmet need for effective treatment options for Barth syndrome. However, lack of approved drugs specifically for the disease along with high cost of therapy are major challenges for market growth in the near future. Moreover, limited patient pool and difficulty in recruiting patients for clinical trials also act as bottlenecks.
Leading market players are engaged in developing new drugs and treatment solutions through partnerships and collaborations to tap growth prospects. Increasing focus on cell and gene therapies also presents new avenues. Emergence of orphan drugs specifically for Barth syndrome and fast track approvals for ongoing clinical trials are key trends observed in the market.
Market Challenge - High costs associated with treatment
One of the major challenges faced by the global Barth syndrome treatment market is the high costs associated with treating the rare genetic condition. Barth syndrome requires lifelong medical care and therapeutic interventions. Many patients need to rely on medication, supplements, dietary modifications, cardiac monitoring, and screening, physical therapy, and psychosocial support throughout their lives. The pharmaceutical treatments available are often very expensive and not affordable for many patients. Further, medical costs continue to rise with advancement in treatment options. The frequent hospital visits and tests also contribute to the growing financial burden on patients. The rarity of the disease poses additional cost hurdles as the small patient pool makes it difficult to recoup development costs of new drugs and therapies. Overall, the high treatment expenditure acts as a major barrier limiting access to quality care and adversely impacting the market's growth potential.
Market Opportunity - Strategic Collaboration and Partnerships for Market
One of the key opportunities for players in the global Barth syndrome treatment market lies in forming strategic collaborations and partnerships. Due to the orphan drug status and small profitable market, pharmaceutical companies are hesitant to independently invest in research and development of new drugs. However, collaborating with patient advocacy groups, medical technology firms, and other stakeholders can help pool resources and expertise. Partnerships enable cost-effective drug discovery and expedite clinical trials. They also facilitate resources sharing, risk distribution, and co-marketing of approved products. Linking with leading hospitals and clinics worldwide provides access to specialized clinical data. Additionally, collaborating with payers helps negotiate reimbursement and patient assistance. If leveraged judiciously, such partnerships can accelerate product commercialization, expand customer outreach, and aid market expansion in the coming years.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
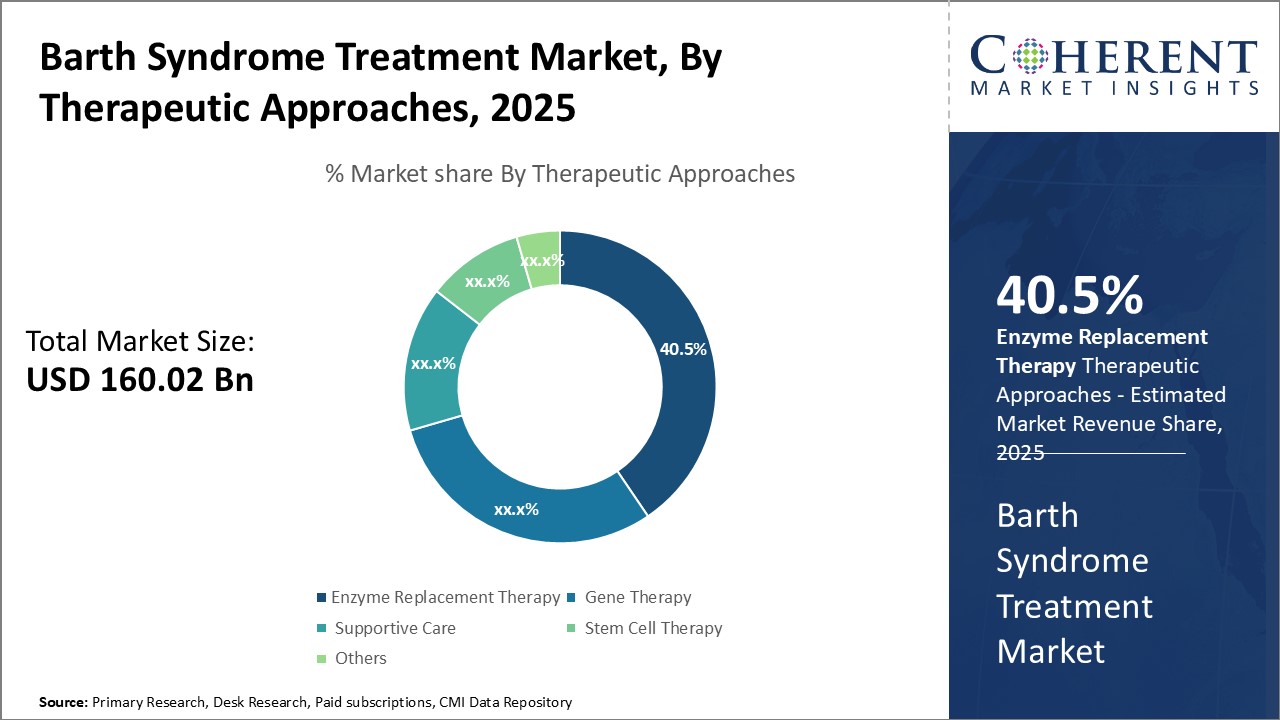
By Therapeutic Approaches- Advances in Research Drive Demand for Enzyme Replacement Therapy
In terms of Therapeutic Approaches, the Enzyme Replacement Therapy segment is estimated to hold the highest share of 40.5% in 2025 owing to ongoing advancements in research. Barth syndrome is caused by a genetic mutation that prevents the body from producing sufficient amounts of an enzyme called tafazzin. This enzyme plays a key role in the mitochondria by remodelling cardiolipin, an important phospholipid that maintains membrane structure and function. Without adequate tafazzin, cardiolipin composition and levels become abnormal, impairing cellular energy production and leading to symptoms.
Enzyme replacement therapy seeks to supplement patients' bodies with the missing tafazzin enzyme through recombinant gene technology. Several investigational therapies are in development that aim to synthesize tafazzin in vitro and deliver it systemically. One such candidate is ENS-100, a rhTafazzin enzyme produced in yeast cells that is being evaluated in an ongoing Phase I/II clinical trial. Interim results have shown the therapy to be well-tolerated with no serious adverse effects reported. Researchers will continue monitoring patients for signs of cardiolipin restoration and improvement in metabolic and cardiac parameters.
Another promising approach involves genetic modification of mouse embryonic stem cells to produce human tafazzin, which are then transplanted into patient tissues. Early animal studies demonstrate these engineered stem cells have the potential to engraft long-term and continuously secrete functional tafazzin. Avoiding repetitive dosing makes this a more practical strategy for lifelong management compared to periodic enzyme infusions. Driven by ongoing basic research progress, enzyme replacement looks poised to become an established treatment option for Barth syndrome in the coming years.
By Drug Class- Cardiolipin Precursors Dominate due to Pivotal Role in Disease Pathogenesis
In terms of Drug Classes, the Cardiolipin Precursors segment is estimated to hold the highest share of 42.6% in 2025 owing to their pivotal role in Barth syndrome pathogenesis. As mentioned earlier, cardiolipin remodelling is disrupted in Barth syndrome due to tafazzin deficiency. Supplementing precursors that feed into cardiolipin biosynthesis aims to bypass the enzyme block and boost endogenous cardiolipin production through alternative pathways.
Tetracycline antibiotics like doxycycline are often prescribed due to their ability to stimulate cardiolipin biosynthesis. Through binding mitochondrial proteins, tetracyclines can activate compensatory pathways that divert metabolic intermediates towards cardiolipin formation. Short-chain phospholipids normally present in people with Barth syndrome have also shown promise. Exogenous phosphatidylglycerol administration in animal studies led to increases in mature cardiolipin, proving the concept of precursor supplementation.
Another prevalent approach involves oral glycerol supplements. Glycerol is converted to glycerol-3-phosphate in the body, an important precursor that feeds into cardiolipin synthesis. Studies indicate glycerol therapy alone or combination with doxycycline can restore cardiolipin levels in fly and nematode Barth syndrome models. Due to their central mechanistic role and proven efficacy, cardiolipin precursors will continue occupying the lion's share of the drug class segment for Barth syndrome management.
By Route of Administration- Oral Route Accounts for the Highest Share Due to Ease and Safety
In terms of Route of Administration, Oral treatment is estimated to hold the highest share of 58.5% in 2025 owing to the ease, safety, and patient acceptability associated with this route. Enzyme replacement and cardiolipin precursor therapies mentioned earlier mostly employ the oral route of delivery due to various advantages. Oral ingestion negates the need for frequent infusions or injections associated with intravenous options. This translates to improved adherence, less burden on healthcare infrastructure and reduced costs.
Oral administration also bypasses vascular delivery challenges as the gut environment provides a larger surface area for drug absorption from the gastrointestinal tract. Metabolic precursors in particular are well-suited to oral dosing since they undergo enteric absorption and first-pass liver metabolism before interacting with target mitochondrial pathways systemically. From a patient perspective, oral therapies are also more discreet and less invasive than alternative procedures.
The safety profile of the oral route is further strengthened through gradual increases in drug levels upon each intake. This allows the body to better adapt compared to instantaneous systemic exposure from injections. With oral therapies dominating current Barth syndrome treatment approaches, this segment is anticipated to be the frontrunner due to intrinsic benefits over other administration types.
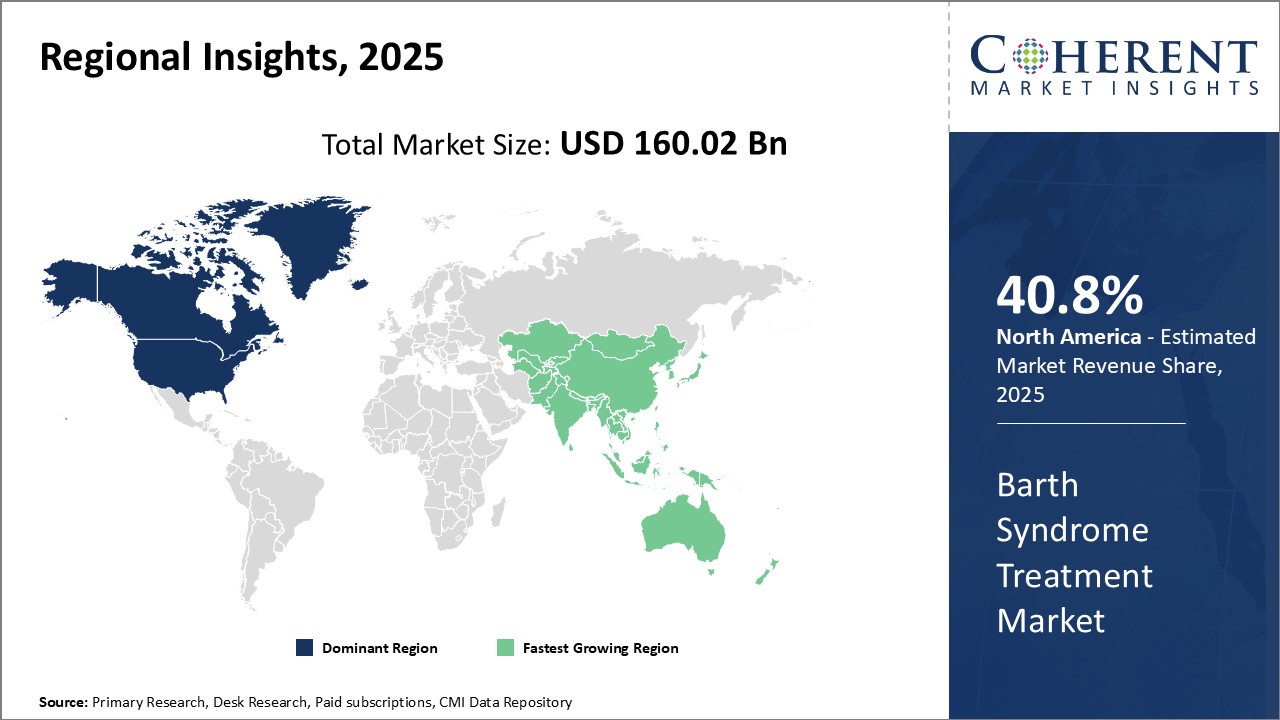
Regional Insights
Need a Different Region or Segment? Download Free Sample
North America has dominated the global Barth syndrome treatment market with an estimated share of 40.8% in 2025 and is expected to continue its leadership position during the forecast period. This can be attributed to the strong presence of leading biotechnology and pharmaceutical companies in countries like the U.S. and Canada who are extensively focused on R&D activities for developing novel treatment options for this rare genetic disease. Additionally, higher healthcare spending and favourable reimbursement policies have enabled improved access to advanced treatment modalities like stem cell therapy and gene therapy in the region.
The Asia Pacific market is anticipated to witness the fastest growth in the coming years. Several factors are driving the market growth in the region. Countries like China, India, Japan and South Korea are witnessing increased diagnosis rates due to improving healthcare infrastructure and growing patient awareness about rare conditions. This has augmented the demand for Barth syndrome specific medications in Asia Pacific. Moreover, the growth of medical tourism industry in Asia with reduced treatment costs compared to developed nations is attracting patients from other global markets for availing superior care.
The European market is also growing steadily supported by rising healthcare expenditures of countries and continuous funding from the European Commission for rare disease research. Key market players are actively engaged in partnerships with hospitals and research institutes to conduct clinical trials and new product launches which are further expanding their regional presence. While the high cost of specialty medications remains a challenge, patient assistance initiatives by pharmaceutical vendors have eased access in Europe.
The growing healthcare needs of Latin America create opportunities for market players. Initiatives by governments towards universal health coverage and investments for strengthening primary care systems offer promising prospects. In addition, the competencies of local generic drug manufacturers in formulation development complement brand name drug availability, rendering the market competitive. Ongoing trade agreements aim to reduce tariffs and capitalize on low manufacturing costs which can facilitate greater export activity.
Market Report Scope
Barth Syndrome Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 160.02 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.4% | 2032 Value Projection: | USD 386.13 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Amneal Pharmaceuticals, Boehringer Ingelheim, Merck & Co., Abbott, B Braun, Integra LifeSciences, Johnson & Johnson, Medtronic, MicroPort Scientific, NuVasive, Orthofix, Perrigo Company, Stryker, Zimmer Biomet, Eli Lilly and Company, F. Hoffmann-La Roche, Mylan, Stealth BioTherapeutics, Sanofi, and Pfizer |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Barth Syndrome Treatment Industry News
- On April 2024, Stealth BioTherapeutics announced that the FDA had accepted its New Drug Application (NDA) for elamipretide, intended to treat Barth syndrome. The NDA is backed by positive results from the SPIBA-001 Phase 3 study and additional data from the TAZPOWER Part 2 trial. Elamipretide has previously received Fast Track, Orphan Drug, and Rare Pediatric Disease designations.
- In May 2023, Pharmanovia expanded its cardiology portfolio by acquiring licensing rights to elamipretide for Barth syndrome in Europe and MENA through an agreement with Stealth BioTherapeutics. The partnership aims to finalize studies showing elamipretide's potential to improve the quality of life for those with Barth syndrome, a rare and serious condition. Promising results have already been seen in ongoing trials, and the drug has received Orphan Drug designations from both the FDA and EMA, highlighting the unmet need in this area.
*Definition: The Global Barth Syndrome Treatment Market focuses on providing treatment options for Barth syndrome. It includes drugs that treat symptoms such as cardiac complications, skeletal muscle weakness, and neutropenia. Since Barth syndrome is a rare genetic disorder with limited treatment options currently available, the market is focused on research and development of new drugs and therapies.
Market Segmentation
- By Therapeutic Approaches Insights (Revenue, USD Bn, 2020 - 2032)
-
- Enzyme Replacement Therapy
- Gene Therapy
- Supportive Care
- Stem Cell Therapy
- Others (Small Molecule Drugs)
- By Drug Classes Insights (Revenue, USD Bn, 2020 - 2032)
-
- Cardiolipin Precursors
- Antioxidants
- Immunomodulators
- Antibiotics
- Dietary Supplements
- Others
- By Route Of Administration Insights (Revenue, USD Bn, 2020 - 2032)
-
- Oral
- Intravenous
- Others (Intramuscular and Topical)
- By End user Insights (Revenue, USD Bn, 2020 - 2032)
-
- Hospitals
- Specialty Clinics
- Research Institutions
- Others
- By Regional Insights (Revenue, USD Bn, 2020 - 2032)
-
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Amneal Pharmaceuticals
- Boehringer Ingelheim
- Merck & Co.
- Abbott
- B Braun
- Integra LifeSciences
- Johnson & Johnson
- Medtronic
- MicroPort Scientific
- NuVasive
- Orthofix
- Perrigo Company
- Stryker
- Zimmer Biomet
- Eli Lilly and Company
- Hoffmann-La Roche
- Mylan
- Stealth BioTherapeutics
- Sanofi
- Pfizer
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
