Baculovirus Expression System Market is estimated to be valued at USD 436.0 Mn in 2025 and is expected to reach USD 781.8 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 8.7% from 2025 to 2032. A baculovirus expression system is a vector-based eukaryotic protein expression system that utilizes an engineered baculovirus to produce recombinant proteins in insect cells. This system provides high protein production yields and correct post-translational modifications for functional protein studies. The key advantages of this system includes high expression levels, proper protein folding and post-translational modifications, rapid generation of vectors, serum-free media capability, and safety.
The key drivers fueling the growth of the baculovirus expression system market include the rising demand for protein therapeutics and vaccines, increasing R&D expenditure and government funding, and technological advancements in expression systems. The market is segmented based on product type, application, end user, and region. Based on product type, the recombinant baculovirus segment accounts for the largest market share owing to the high efficiency and versatility of recombinant baculoviruses for protein expression.
Baculovirus Expression System Market Regional Insights:
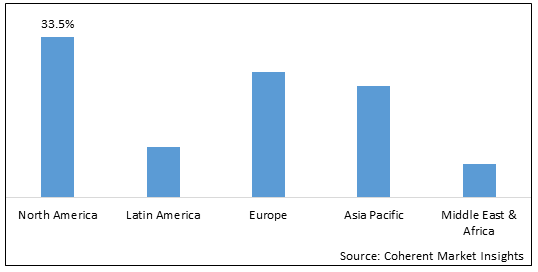
- North America is expected to be the largest market for baculovirus expression systems during the forecast period, which accounted for over 33.5% of the market share in 2025. The growth of the market in North America is attributed to the strong presence of leading biopharmaceutical companies, significant R&D investments, and rapid adoption of advanced technologies.
- Europe is expected to be the second-largest market for baculovirus expression systems, which accounted for over 25.1% of the market share in 2025. This is due to the increasing demand for recombinant proteins from the pharmaceutical and biotechnology industries in the region.
- Asia Pacific is expected to be the fastest-growing market for baculovirus expression systems, with a CAGR of over 19.2% during the forecast period. The growth of the market in Asia Pacific is attributed to the increasing demand for protein therapeutics, rising investments in R&D infrastructure, and growing outsourcing of bio production by global biopharmaceuticals
Figure 1. Global Baculovirus Expression System Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Analyst’s View on Baculovirus Expression System Market
The market for the Baculovirus expression system is expected to grow significantly in the coming years. This growth is attributed to the increasing application of the baculovirus expression vector system in vaccine development. The rising demand for protein therapeutics and vaccines, increasing R&D expenditure, and technological advancements in expression systems are key drivers fueling this growth. However, the complexity and length of the BEVS process may discourage some potential users. The market is segmented by product type, application, end-user, and region, offering diverse opportunities for stakeholders. Overall, the analyst view underscores the potential and versatility of the baculovirus expression system in meeting the growing demand for protein therapeutics and vaccines.
Baculovirus Expression System Market Drivers:
- Increasing Demand for Protein Therapeutics and Vaccines: The rising prevalence of chronic diseases such as cancer, autoimmune disorders, and infectious diseases is driving the demand for new therapeutic proteins and vaccines. Pharmaceutical companies are increasingly adopting the baculovirus expression system for the production of complex protein therapeutics as it provides proper post-translational modifications and protein folding critical for functionality. Several blockbuster biologic drugs including monoclonal antibodies and gene therapy vectors utilize baculovirus expression. Moreover, the COVID-19 pandemic has highlighted the need for rapid, scalable manufacturing platforms for vaccines and therapeutics. The baculovirus-insect cell system allows the quick production of vaccine antigens to respond to emerging pandemic threats. For instance, according to Springer Journal of Genetic Engineering & Biotechnology, publication ‘Application of Baculovirus Expression Vector system (BEV) for COVID-19 diagnostics and therapeutics’ in July, 2022, Thousands of proteins have been successfully created using the BEV platform, and it is no longer limited to research purposes; it is now being used on a broad scale to manufacture a variety of biological products, including vaccines. At first, only two Baculovirus Expression Vector (BEV)-derived vaccines were commercialized. Since then, numerous products have been approved for usage including the latest Novavax’s COVID-19 vaccine.
- Technological Advancements in Expression Systems: Key players are investing significantly in R&D to develop improved baculoviral vectors, serum-free media formulations, high-performance cell lines, and automated bioprocessing technologies. For instance, advancements such as flashBACTM and BaculoJuiceTM system enable the rapid generation of high-titer viral stocks leading to increased protein yields. Moreover, innovations in instrumentation such as the introduction of single-use bioreactors provide advanced process controls and scalability. Such technological advancements are making baculovirus expression more efficient, versatile, scalable, and cost-effective for biopharmaceutical production.
- Increasing Strategic Collaborations and Mergers for the Development of Advanced Expression Systems: The increasing investments by rising strategic collaboration and merger activities by key players with emerging players of in life science is increasing research and development activities as well as biopharmaceutical firms to accelerate drug discovery is driving the growth of the baculovirus expression system market. Moreover, government funding and incentives to promote the development of protein therapeutics, vaccines, and biosimilars is expected to present significant growth opportunities. Expanding application areas into emerging fields, such as gene therapy and synthetic biology, will also boost the adoption of Baculovirus expression technologies. For instance, in January 2022, VectorY, a Netherlands-based biotech company dedicated to developing transformative medicines, announced its collaboration with Wageningen University & Research Center to develop novel Baculovirus-based technologies for the production of safe and affordable Adeno-associated viruses (AAV) gene therapies. This partnership will further strengthen VectorY’s proprietary AAV production technologies for the development of next generation gene therapies.
Baculovirus Expression System Market Opportunities:
- Increasing Adoption in Vaccine Development: The COVID-19 pandemic has highlighted the need for agile, scalable vaccine production platforms. The baculovirus-insect cell system allows rapid, economical manufacturing of safe and effective subunit vaccines. With the ability to produce properly folded complex antigens, this system has gained increasing adoption in development of viral vaccines including influenza, hepatitis B, SARS-CoV-2, and others. Moreover, baculovirus is suitable for the production of virus-like particles, which presents target antigens more accurately to the immune system. Therefore, the expanding application for vaccine development presents significant opportunities. For instance, the World Health Organization (WHO) reported in 2021 that over a dozen manufacturers have capabilities to express recombinant proteins using baculovirus for potential COVID-19 vaccines. Overall, increasing investments in vaccine research from public health organizations provide a great opportunity for the baculovirus expression systems to contribute significantly to global immunization efforts in the coming years.
- Growth in Gene Therapy and Viral Vectors: The baculovirus expression system is gaining traction for the manufacturing of gene therapy vectors including adeno-associated viruses (AAV). It allows high-yield, scalable production of rAAV vectors carrying therapeutic genes. With several gene therapy products getting approved and strong clinical pipelines, the rising demand for viral vectors production will drive the adoption of the baculovirus platform. Key players are also making significant investments to leverage baculovirus expression for efficient, economical manufacturing of gene therapies. For instance, the US NIH (National Institute of Health) invested over US$ 644 million in fiscal year 2021 for gene therapy research according to its funding data. The European Commission too allocated US$ 342.2 million toward new gene and cell therapy initiatives indicative growing support and investments into gene therapies by global government bodies will likely fuel greater demand for reliable, scalable and economical viral vector manufacturing using the Baculovirus expression platform. As clinical translation of novel gene therapies ramps up in the coming years to address various genetic disorders, the Baculovirus expression system is anticipated to play a strategic role in supporting their large-scale good manufacturing needs.
Baculovirus Expression System Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 436.0 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.7% | 2032 Value Projection: | USD 781.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Thermo Fisher Scientific, Merck KGaA, Takara Bio, Agilent Technologies, Oxford Expression Technologies, Promega Corporation, Qiagen N.V., Bio-Rad Laboratories, New England Biolabs, Genscript Biotech Corporation, Syngene International Limited, Sartorius AG, Aragen Bioscience, Vivopure, Creative Biogene, Absolute Antibody, Rockland Immunochemicals, Protein Technologies Inc., Proteogenix, and Virovek |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Baculovirus Expression System Market Trends:
- Transition to Serum-free Insect Cell Culture: Earlier insect cell cultures required animal-derived sera for growth and productivity. However, concerns with variability, contamination risks, and ethical issues are driving a shift towards serum-free media. Key players are developing chemically-defined, animal component-free media supporting high-density suspension cultures. Serum-free systems enable more consistent, scalable, and GMP (Good Manufacturing Practices)-compliant baculovirus protein expression. This represents a promising trend that is expected to continue gaining momentum. For instance, according to an article published in Frontiers journal ‘Genetic engineering of baculovirus-insect cell system to improve protein production’ in September 2022, as of now, 11 BEVS-derived products have been approved for use including four human vaccines [Cervarix against cervical cancer caused by human papillomavirus (HPV), Flublok and Flublok Quadrivalent against seasonal influenza, Nuvaxovid/Covovax against COVID-19], two human therapeutics [Provenge against prostate cancer and Glybera against hereditary lipoprotein lipase deficiency (LPLD)] and five veterinary vaccines (Porcilis Pesti, BAYOVAC CSF E2, Circumvent PCV, Ingelvac CircoFLEX and Porcilis PCV). The BEVS has many advantages, including high safety, ease of operation, and adaptable for serum-free culture.
- Automation and Single-use Technologies: Companies are incorporating automation and single-use technologies to improve process efficiencies, increase productivity, and reduce costs. Automated systems for cell culture seeding, infection, harvesting minimize manual labor and chances of contamination. Prefabricated, single-use bioreactors with advanced sensors enable continuous processing. Implementation of disposable technologies also minimizes validation between production batches. These trends are enhancing the versatility and adoption of baculovirus expression.
Baculovirus Expression System Market Restraints:
- Production Costs: While the baculovirus system provides high expression yields, the overall production costs remains relatively high. Requirement of costly media, proteins, enzymes, and need for maintaining aseptic bioreactor conditions contribute to high costs which may hinder the adoption by small R&D firms and academic institutes. Lack of cost-effective scale-up strategies also restrain the market growth. For instance, according to data published by the United Nations International Labour Organization in 2021, the average wage of skilled biotechnicians and process engineers needed for insect cell culture work is around US$ 35-45 per hour in the U.S. and major European countries. Considering the intensive labor involved, personnel costs are a major contributor to the overall production expenses. This makes baculovirus expression less favorable for research and production of low cost therapies. Unless the production costs are significantly reduced by innovations in culture and downstream processing methods, the technology will see slower commercial uptake.
- Risk of Contamination: The use of live baculoviruses and serum-containing media leads to risks of contamination by adventitious viruses and prions. This raises safety concerns and chances of product rejection, resulting in loss of expensive reagents and bioprocessing materials. Implementing proper biosafety controls and serum-free systems can help mitigate these risks. For instance, according to the publication in Eureka Journal, ‘A New Way of Gauging Baculovirus Residue in Gene Therapy Products’ published in August, 2022, Baculovirus is the high risk of adventitious and endogenous virus contamination. During production, a high level of residual baculovirus from the host cell sometimes remains in the intermediate and final product. Although not fatal to humans, if left undetected the residual baculovirus has the potential to cause inflammation and other immunogenic responses in patients.
Recent Developments:
New product launches:
- In February 2023, Thermo Fisher Scientific, a U.S.-based supplier and analytical instruments, life sciences solutions, specialty diagnostics, laboratory, pharmaceutical, and biotechnology services company, announced launch of the BaculoDirect Baculovirus Expression System, which is a streamlined system for the production of recombinant proteins in insect cells. The system includes a new vector, transferrin receptor-mediated transfection (TRMT) technology, and a new Sf9 insect cell line.
- In March 2023, Oxford Expression Technologies, a K.-based biotechnology company, launched the AcMNPV BaculoDirect Expression System, which is a system for the rapid and efficient production of recombinant proteins in insect cells. The system includes a new vector, a new insect cell line, and a transfection reagent.
- In April 2023, Promega Corporation, a U.S.-based manufacturer of enzymes and other products for biotechnology and molecular biology company, launched the pFastBac Dual Baculovirus Expression System, which is a system for the production of two recombinant proteins in insect cells from a single vector. The system includes a new vector, a new insect cell line, and a transfection reagent.
Acquisitions and partnerships:
- In April 2022, Thermo Fisher Scientific, a U.S.-based supplier of analytical instruments, life sciences solutions, specialty diagnostics, laboratory, pharmaceutical, and biotechnology services, partnered with Watchmaker Genomics, is a biotechnology company that focuses on breakthrough applications for reading, writing, and editing DNA. To improve AAV production for gene therapy using the Thermo Fisher Baculovirus Expression Vector System.
- In June 2021, Aragen Bioscience, a global leader in providing drug discovery, drug development, and manufacturing solutions for life sciences, acquired Exelead's CMO business to expand its biologics and viral vector CDMO capabilities including Baculovirus-insect cell expression platforms.
- In May 2020, Sartorius AG, a global laboratory and process technology provider for the biotech, pharmaceutical, and food industries, acquired a majority stake in cell culture media expert biological industries. This enhanced Sartorius AG' expertise in insect cell culture media for baculovirus expression.
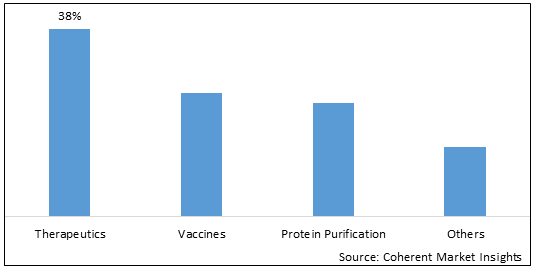
Figure 2. Global Baculovirus Expression System Market Share (%), by Applications, 2025
To learn more about this report, Download Free Sample
Top Companies in the Baculovirus Expression System Market:
- Thermo Fisher Scientific
- Merck KGaA
- Takara Bio
- Agilent Technologies
- Oxford Expression Technologies
- Promega Corporation
- Qiagen N.V.
- Bio-Rad Laboratories
- New England Biolabs
- Genscript Biotech Corporation
- Syngene International Limited
- Sartorius AG
- Aragen Bioscience
- Vivopure
- Creative Biogene
- Absolute Antibody
- Rockland Immunochemicals
- Protein Technologies, Inc. (PTI)
- Proteogenix
- Virovek
Definition: The baculovirus expression system is a powerful platform for producing recombinant proteins in insect cells infected with genetically engineered baculoviruses. It utilizes the strong viral promoters of the baculovirus to drive high levels of protein expression in insect cell lines. In this system, the gene of interest is cloned into a baculovirus shuttle vector. This recombinant transfer vector is then used to generate recombinant baculoviruses capable of entering and expressing the target protein in insect cells. The infected insect cells then become little "factories" churning out large amounts of the recombinant protein for purification and characterization.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




