Atezolizumab Market is estimated to be valued at USD 3.64 Bn in 2025 and is expected to reach USD 10.67 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 16.6% from 2025 to 2032. Atezolizumab is an anti-PD-L1 monoclonal antibody used for treating various types of cancers. It helps activate the immune system to attack and kill cancer cells. Atezolizumab is administered intravenously, either alone or in combination with other anti-cancer drugs. Key drivers include the rising prevalence of cancer globally, increasing pharmaceutical R&D, and favorable reimbursement policies in developed nations.
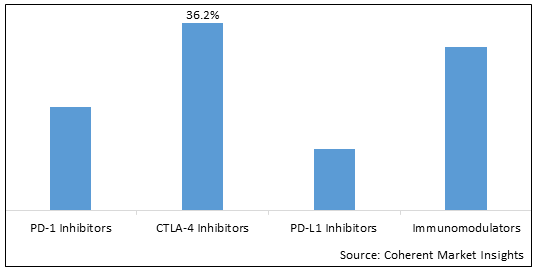
The Global Atezolizumab Market is segmented by drug class, indication, and distribution channel, end user, and region. By drug class, the PD-L1 inhibitors segment is expected to account for the largest share during the forecast period. PD-L1 inhibitors like atezolizumab have shown improved efficacy in a wide range of cancers, fueling their high adoption.
Global Atezolizumab Market Regional Insights
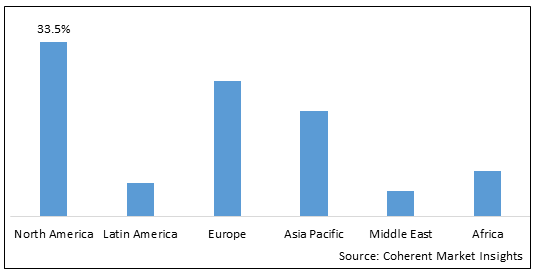
- North America is expected to be the largest market for atezolizumab during the forecast period, accounting for over 33.5% of the market share in 2025. The growth of the market in North America is attributed to rising cancer prevalence, new product launches, high healthcare spending, and favorable reimbursements in the region.
- Europe is expected to be the second-largest market for atezolizumab, accounting for over 26.2% of the market share in 2025. The growth of the market in Europe is attributed to the availability of research funding, presence of leading pharma giants, and increasing clinical trials evaluating atezolizumab.
- Asia Pacific is expected to be the fastest-growing market for atezolizumab, with a CAGR of over 17.1% during the forecast period. The growth of the market in Asia Pacific is attributed to the rising disposable incomes, healthcare infrastructure modernization, and targeted expansion efforts by leading players in the region.
Figure 1. Global Global Atezolizumab Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Analyst’s View on Global Atezolizumab Market
Atezolizumab is a promising immunotherapy drug that can treat several types of cancer. As an immune checkpoint inhibitor, it works by blocking PD-L1 and allowing T-cells to kill cancer cells more effectively. North America is likely to dominate the market given early FDA approval and strong reimbursement system. However, manufacturing and supply chain challenges can restrain growth in the short term. Asia Pacific is expected to be the fastest growing region due to rising healthcare spending and increasing incidence of cancer in countries like China and India.
The drug has already gained FDA approval for urothelial carcinoma and non-small cell lung cancer which are two large cancer markets. Further approvals for renal cell carcinoma and triple negative breast cancer will drive additional growth. Combination therapies with chemotherapy or radiation can widen the commercial potential. Ongoing trials for other cancers including head and neck, gastric and hepatocellular carcinoma signal future opportunities. Competition from other checkpoint inhibitors like Pembrolizumab is a threat, although the addressable market is big enough for multiple drugs. Pfizer's partnership with Merck for co-development and co-commercialization in various markets will aid in maximizing revenue. Patient assistance programs will help address affordability concerns in the long run. Favorable side effect profile compared to chemotherapy increases patient acceptance and compliance to the treatment.
Global Atezolizumab Market Drivers:
- Rising prevalence of cancer globally: Cancer is one of the leading causes of death worldwide, According to Center for Disease Control and Prevention (CDC) with approximately 19.3 million new cases and 10.0 million cancer deaths in 2020 alone. The global burden of cancer is expected to grow to 30.2 million new cases and 16.4 million deaths by 2040 simply due to the growth and aging of the population. The rising prevalence of various cancers such as lung cancer, melanoma, bladder cancer, and head and neck cancers is driving demand for novel therapies like atezolizumab. Atezolizumab is an anti-PD-L1 monoclonal antibody that helps activate the immune system to attack and kill cancer cells. The growing number of cancer cases across the globe is fueling the uptake of immunotherapies like atezolizumab, thereby driving market growth.
- Increasing pharmaceutical R&D for cancer immunotherapy: Pharmaceutical companies are aggressively focusing on R&D for novel cancer immunotherapies given their potential to significantly improve patient outcomes. There is increasing investment in developing combinatorial immunotherapies using immune checkpoint inhibitors like atezolizumab along with chemotherapy, targeted therapies, or other immuno-oncology agents. For instance, Roche has an extensive clinical program evaluating atezolizumab in combination with other novel molecules for treating various cancers. Moreover, companies are developing biosimilar versions of atezolizumab to expand access. The rapid pace of innovation and clinical research for atezolizumab and other immunotherapies is expected to widen the array of treatment options available and propel market growth. According to Cancer Research Institute’s article ‘Cellular Cancer Immunotherapy Development Evolves, Expands with New Technologies and Targets’ published in June, 2022, in April, there were 2,756 active cell therapy agents in the global immuno-oncology pipeline, an increase of 36% over the 2021 landscape analysis that identified 2,031 such agents, but also a modest deceleration compared to 43% growth in the prior year.
- Rising healthcare expenditure globally: The gradual increase in healthcare spending globally, especially in developing countries, is contributing to the increasing adoption of high-cost cancer therapies like atezolizumab. According to the World Health Organization, report of year 2022, global healthcare expenditure is projected to reach US$ 10 trillion by 2022, indicating rising budgets and health system capacity to adopt novel treatment options. Moreover, the rapid economic growth in emerging countries like India and China is boosting healthcare spending. The rising healthcare investments will drive greater drug accessibility, accelerate uptake of immunotherapy in developing markets, and fuel the Global Atezolizumab Market growth.
Atezolizumab Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.64 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 16.6% | 2032 Value Projection: | USD 10.67 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Roche, Novartis, Pfizer, Merck, AstraZeneca, Bristol-Myers Squibb, BeiGene, Innovent Biologics, Genentech, AbbVie |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Atezolizumab Market Opportunities:
- Leveraging partnerships and acquisitions: Partnerships and acquisitions offer attractive opportunities for companies in the Global Atezolizumab Market to accelerate R&D pipelines, expand into emerging markets, and consolidate market position. There have been several strategic deals among pharmaceutical giants to maximize the value of their immuno-oncology portfolios. For instance, in 2020, Merck, Germany based Global Pharmaceutical company and Seagen, U.S. based Biotechnology Company, partnered to co-develop therapies combining Keytruda and ladiratuzumab vedotin. Such collaborative agreements allow companies to complement their expertise. Moreover, licensing deals with local players can aid market expansion in high-potential regions. Capitalizing on strategic tie-ups and M&A will be an impactful opportunity.
- Exploring combination therapies: An emerging opportunity is the development of novel combination treatments using atezolizumab and other anti-cancer drugs like chemotherapy, targeted therapy, or radiation therapy. Combination regimes allow complementary action on tumors to improve patient outcomes through synergistic effects. For instance, on January 18, 2023, Genentech, a U.S.-based biotechnology company, announced the Pivotal Phase III IMbrave050 study investigating Tecentriq plus Avastin in people with early-stage hepatocellular carcinoma (HCC) at high risk of recurrence following surgery met primary endpoint of recurrence-free survival. In addition of Avastin to Tecentriq led to increased overall survival in liver cancer patients. Companies can further their clinical research on pairing atezolizumab with different mechanisms to provide more potent and durable responses. Gaining regulatory approvals for effective combination therapies will significantly boost commercial potential.
Global Atezolizumab Market Trends:
- Adoption of biosimilars: The expiration of patent exclusivity periods for monoclonal antibody therapies like atezolizumab is driving the development and approval of biosimilars. Industry leading pharmaceutical companies are focusing R&D on bringing low-cost biosimilars of atezolizumab and other immuno-oncology drugs to market. These follow-on biologics provide cost savings for patients and healthcare systems. For instance, Amgen’s biosimilar of Avastin gained U.S. Food and Drug Administration (FDA) approval in 2020. The launch of biosimilars eats into branded market share but expands the overall patient population benefiting from atezolizumab therapy. More regulatory approvals and uptake of biosimilars will be an influential trend.
- Combination therapies gaining prominence: Using immunotherapy agents like atezolizumab in combination therapy regimens is rising as an integral trend in cancer care. Concurrent administration of atezolizumab with chemotherapy, targeted drugs, or other immuno-oncology agents allows complementary mechanisms of action. Combinations demonstrate substantial improvements in tumor response and patient survival compared to monotherapies. For instance, in October 2021, Genetec, a U.S. based Biotechnology Company, announced the U.S. Food and Drug Administration approval for atezolizumab for adjuvant treatment following resection and platinum-based chemotherapy in patients with stage II to IIIA non-small cell lung cancer (NSCLC).
- Next-generation immunotherapies: As research continues, next-generation immuno-oncology agents are starting to emerge with potential advantages over atezolizumab. These include bispecific T cell engager antibodies, cytokine therapies, and cell therapies like CAR-T. Novel modalities offer opportunities to enhance anti-tumor immunity. Pharmaceutical players are investing significantly in the next wave of innovation. While development is still ongoing, thefuture availability of these advanced immunotherapies could impact atezolizumab’s dominance and represent disruption. However, atezolizumab biosimilars could balance cost pressures and sustain volumes.
Global Atezolizumab Market Restraints:
- High cost of treatment: Despite their profound efficacy, atezolizumab and other cancer immunotherapies come at a very high price, creating affordability challenges that restrain wider adoption. The cost burden on patients and payers has risen with accumulating U.S Food and Drug Administration (FDA) approvals for atezolizumab across numerous indications. For instance, according to Lung cancer journal publication in July 2023, the list price of Tecentriq is over US$ 15,000 per month. While insurance coverage is helping patients in developed countries gain access, those in emerging economies face stark access barriers due to high costs. Addressing the pricing and reimbursement hurdles will be vital for market expansion.
- Immune-related adverse effects: As a downside of immune system stimulation, atezolizumab therapy poses risks of immune-related adverse events which can diminish quality of life. Reported side effects include colitis, hepatitis, endocrinopathies, pneumonitis, and nephritis. Severe irAEs often require treatment discontinuation or immunosuppressive therapy. Safety concerns due to side effects thus limit uptake of atezolizumab. However, research is elucidating potential risk biomarkers and mitigation strategies to allow safer administration. For instance, On February 2023, according to JAMA oncology article ‘Association of Immune-Related Adverse Events With Efficacy of Atezolizumab in Patients With Non–Small Cell Lung Cancer’, Immune checkpoint inhibitors (ICIs) increase antitumor activity by blocking downregulators of the immune system and have transformed the treatment and prognosis of lung cancer. ICIs are associated with off-target immune and inflammatory adverse effects known as immune-related adverse events (irAEs).
Recent Developments
New product launches
- In March 2022, Roche, Swiss bases multinational healthcare company, launched a new subcutaneous formulation of Tecentriq for treating non-small cell lung cancer. The formulation provides flexibility and reduces administration time.
- In June 2021, BeiGene, global biotechnology company that is discovering and developing innovative oncology treatments announced the approval of its PD-1 inhibitor tislelizumab in China for treating previously treated locally advanced or metastatic esophageal squamous cell carcinoma. This expanded tislelizumab’s applicability.
- In April 2021, Bristol Myers Squibb, U.S based multinational pharmaceutical company, received the U.S. Food and Drug Administration (FDA) approval for Opdivo (nivolumab) plus Yervoy (ipilimumab) combined with limited chemotherapy for treating metastatic or recurrent non-small cell lung cancer. This combination regime improved overall survival.
Acquisition and partnerships
- In October 2022, Seagen, U.S. based Biotechnology Company, and RemeGen, global biopharmaceutical company, focusing on the discovery, development and commercialization of novel biologics, announced a collaboration agreement to develop disitamab vedotin, an antibody-drug conjugate, in Asia Pacific and other regions.
- In December 2021, Novartis, U.S. based global Pharmaceutical Company, acquired Arctos Medical, a spin-off company from the University of Bern and has developed a revolutionary gene therapy, obtaining its rights to an early-stage STING (STimulator of INterferon Gene) agonist program. This enhanced Novartis’ immuno-oncology pipeline.
- In April 2020, Pfizer, Global Pharmaceutical and Biotechnology Company, completed the acquisition of Array Biopharma, a biopharmaceutical company focused on the discovery, development and commercialization of targeted small molecule drugs, strengthening its portfolio of targeted cancer medicines paired with immuno-oncology drugs.
Figure 2. Global Global Atezolizumab Market Share (%), By Drug Class, 2025
To learn more about this report, Download Free Sample
Top Companies in Atezolizumab Market
- Roche
- Novartis
- Pfizer
- Merck
- AstraZeneca
- Bristol-Myers Squibb
- BeiGene
- Innovent Biologics
- Genentech
- AbbVie
Definition: The Global Atezolizumab Market refers to the industry and market associated with the development, production, distribution, and sales of the monoclonal antibody drug atezolizumab. Atezolizumab is an immunotherapy drug used for treating various types of cancer. It works by binding to PD-L1 protein and blocking its interactions with PD-1 and B7.1 proteins on tumor cells and T-cells respectively. This helps restore T-cell activation and boosts the immune system’s ability to detect and destroy cancer cells. Atezolizumab has demonstrated efficacy in cancers such as non-small cell lung cancer, bladder cancer, triple-negative breast cancer and others.
Few Other Promising Reports In Pharmaceutical Industry
Fc Protein And Glycoengineered Antibodies Market
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




