Allogeneic Stem Cell Transplantation Market Size and Forecast – 2025-2032
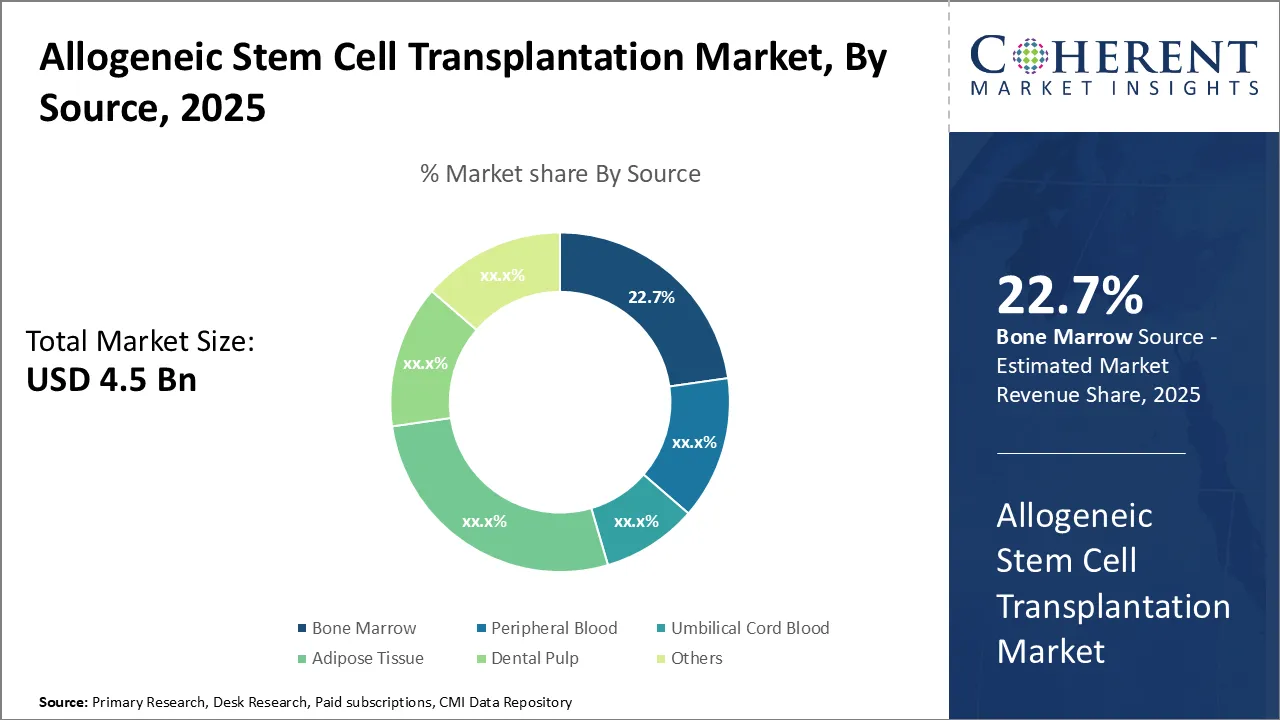
Allogeneic Stem Cell Transplantation Market is estimated to be valued at USD 4.53 Bn in 2025 and is expected to reach USD 7.66 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 7.8% from 2025 to 2032.
Key Takeaways
- Based on source type, the bone marrow segment is dominant, accounting for around 25% of the revenue shares in 2025 owing to the increasing use of bone marrow as a primary source for transplantations.
- Based on Cell Type, the market is bifurcated into Allogeneic MSCs (Allogenic Mesenchymal stem cells), Allogeneic HSCs (Allogeneic hematopoietic stem cells), and Others. The demand for these cell types is attributed to the increasing demand for advanced treatments for hematologic disorders and growing awareness of stem cell therapies.
- Based on Application, the market is segmented into Cancer, Autoimmune Diseases, Blood Disorders, Cardiac Disorders, Neurological Disorders, Diabetes, and Others. The demand is driven by the expanding applications across various therapeutic areas.
- On the basis of End-User, the market is classified into Hospitals, Research Institutes, Specialty Clinics, and Others. The surging demand for advanced treatments in hospitals, research institutes, and specialty clinics is proliferating the allogeneic stem cell transplantation market growth.
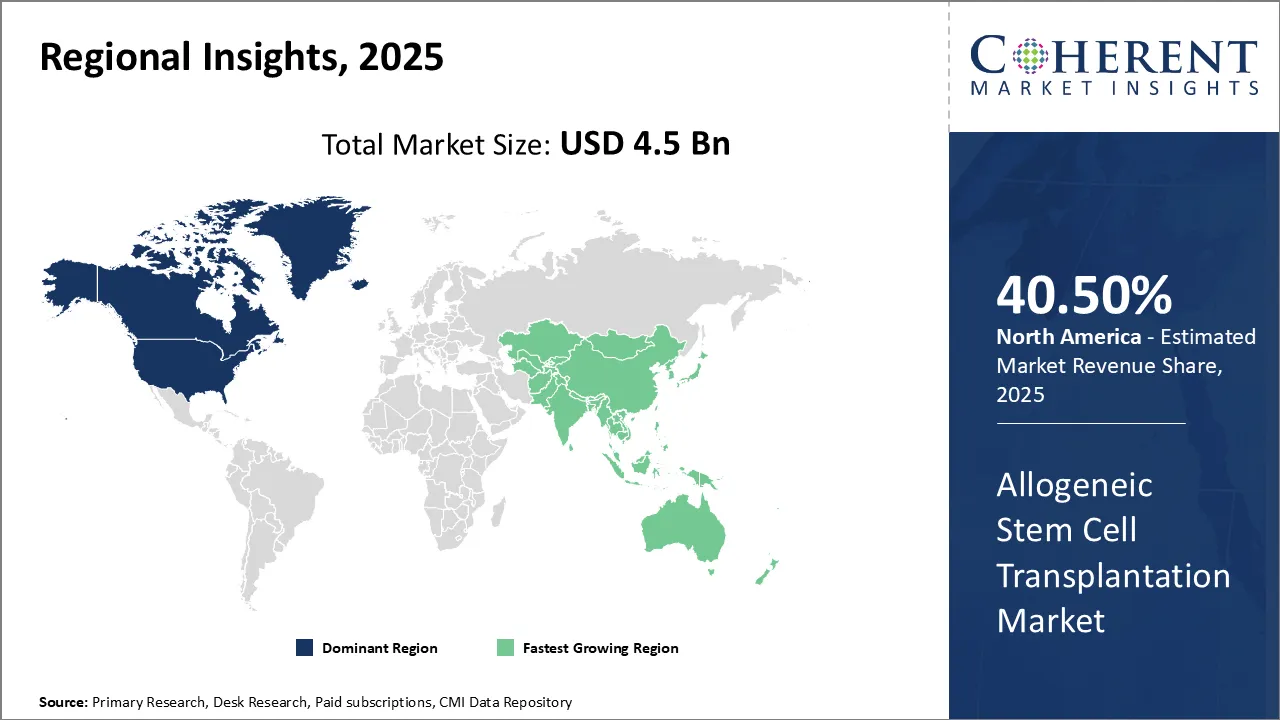
- On the basis of region, North America is dominating the market in 2025, accounting for 40.5% of the overall share owing to the escalating adoption of advanced technologies.
Market Overview
Allogeneic stem cell transplantation involves transferring stem cells from a genetically similar donor to the patient for the treatment of various disorders and cancers. It has key applications in autoimmune diseases treatment and genetic disorders. The rising prevalence of chronic diseases is a key factor driving the market growth.
Current Events and Their Impact
|
Current Events |
Description and its impact |
|
Technological Innovations
|
|
|
Healthcare Infrastructure Developments |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Allogeneic Stem Cell Transplantation Market Insights, by Source- Growing Prevalence of Cancer is driving the Bone Marrow Segment’s Growth
The allogeneic stem cell transplantation market is segmented by source into bone marrow, peripheral blood, umbilical cord blood, adipose tissue, dental pulp, and others. Bone marrow remains a traditional and widely used source, particularly for hematological cancers and inherited disorders. The high success rate of bone marrow transplants, along with its ability to regenerate the immune system and restore normal blood cell production, makes it a preferred choice in clinical settings. For instance, A 2021 study in JAMA Oncology estimates that around 10,000 patients in the U.S. receive allogeneic stem cell or bone marrow transplants each year. Over the past 40 years, the process of bone marrow transplants has improved. Advances in care and treatment have also made it less likely for patients to develop graft-versus-host disease (GVHD), a serious complication of the transplant, further positively influencing the allogeneic cell transplantation market forecast.
Allogeneic Stem Cell Transplantation Market Insights, by Cell Type, the Market is Segmented into Allogeneic Mesenchymal Stem Cells (MSCs), Allogeneic Hematopoietic Stem Cells (HSCs)
Based on cell type, the market is categorized into allogeneic mesenchymal stem cells (MSCs), allogeneic hematopoietic stem cells (HSCs), and others. Allogeneic MSCs are increasingly used in regenerative medicine and immune-related applications due to their immunomodulatory properties and multipotency. Allogeneic HSCs, derived from donor bone marrow, peripheral blood, or cord blood, are extensively used in treating leukemia, lymphoma, and various blood disorders. Also, the National Marrow Donor Program in the U.S. has facilitated thousands of HSC transplants, which have led to increased survival rates for patients with conditions like leukemia.
Allogeneic Stem Cell Transplantation Market Insights, By Application type, the Market is Segmented into Cancer, Autoimmune Diseases, Blood Disorders, Cardiac Disorders, Neurological Disorders, and Diabetes
In terms of application, the market spans cancer, autoimmune diseases, blood disorders, cardiac disorders, neurological disorders, diabetes, and others. Cancer acquires the prominent share, particularly for hematologic malignancies like leukemia and multiple myeloma, where stem cell transplants are often curative. Autoimmune diseases are increasingly being treated with stem cell therapies aimed at resetting the immune system. Blood disorders such as thalassemia and sickle cell anemia also see extensive use of stem cell transplants. Also, in September 2024, Coal India launched the third phase of its Thalassaemia Bal Sewa Yojna, a CSR project. The initiative provides financial support for stem cell transplants to children with thalassemia across India, offering them hope for a transfusion-free, healthier future. Cardiac and neurological disorders are key areas of ongoing research, with stem cells being evaluated for tissue regeneration and repair. In diabetes, stem cells aim to regenerate insulin-producing pancreatic cells. These are further positively influencing the allogeneic stem cell transplantation market revenue.
Allogeneic Stem Cell Transplantation Market Insights, By End User, the Market is Segmented Into Hospitals, Research Institutes, and Specialty Clinics.
By end user, the market includes hospitals, research institutes, specialty clinics, and others. Hospitals are the primary centers for stem cell transplantation, offering integrated treatment facilities under stringent regulatory protocols. For instance, in December 2024, Tampa General Hospital (TGH) launched a new outpatient Cellular-Immunotherapy Transplant Unit at the TGH Cancer Institute, providing alternative cell therapy treatments for patients with aggressive blood cancers like leukemia, lymphoma and multiple myeloma. Research institutes play a vital role in advancing stem cell science through clinical trials and translational research. Specialty clinics, often focusing on regenerative medicine, provide personalized therapies for chronic conditions. The “others” segment comprises biobanks, cell therapy manufacturing units, and contract research organizations (CROs), which support clinical and commercial development in the stem cell field, further propelling the allogeneic stem cell transplantation market share.
Innovation of Advanced Technologies in allogeneic stem cell transplantation market
Significant advances in stem cell harvesting, expansion, and therapeutic application have enhanced the scope of allogeneic stem cell-based treatments. New developments in apheresis for stem cell collection from peripheral blood, the use of cord blood as a source, and advances in ex vivo expansion techniques have improved cell yields and transplantation outcomes. Companies are also developing advanced allogeneic 'off-the-shelf' cell therapies using gene editing tools like CRISPR to make universal CAR T-cell therapies for cancer treatment. Such cutting-edge technologies and treatment modalities will propel the market growth. For instance, in February 2025, Cellistic, a leader in iPSC-based off-the-shelf cell therapy development and manufacturing, announced the introduction of its Allo Chassis™. This innovative offering consists of ready-to-use, immune-protected iPSC cell lines derived from CD34+ and CD4+ T-cell primary cells. This is further accelerating the allogeneic stem cell transplantation market growth.
Regional Analysis
To learn more about this report, Download Free Sample
North America allogeneic stem cell transplantation market trends
North America is expected to be the largest market for allogeneic stem cell transplantation during the forecast period, accounting 40.5% of the market share in 2025. The growth of the market in North America is attributed to the high adoption of stem cell therapies, advanced healthcare infrastructure, and rising R&D investments. In October 2024, OmniaBio Inc. will unveil Canada's largest CGT-focused contract development and manufacturing facility, also serving as a new center of excellence for cell and gene therapy and artificial intelligence.
Europe allogeneic stem cell transplantation market trends
Europe is expected to be the second-largest market for allogeneic stem cell transplantation, which accounted for over 27.2% of the market share in 2025. The growth of the market in Europe is attributed to the increased funding for clinical trials and the growing cases of cancers and blood disorders.
Asia Pacific allogeneic stem cell transplantation market trends
Asia Pacific is expected to be the fastest-growing market for allogeneic stem cell transplantation, growing at a CAGR of over 23.1% during the forecast period. The growth of the market in Asia Pacific is attributed to the growing healthcare expenditure and increasing government support for R&D regarding allogeneic stem cell transplantation. In 2022, Europe saw 4,471,422 new cancer cases, with a cumulative risk of 27.9% by age 75. This is further driving the allogeneic stem cell transplantation market demand.
Allogeneic Stem Cell Transplantation Market Trend
Rising Incidence of Chronic Diseases
The rising prevalence of chronic diseases, such as cancer, autoimmune diseases, and blood disorders, is a major factor driving the growth of the allogeneic stem cell transplantation market. The number of new cases of diseases like leukemia, multiple myeloma, and lymphoma has steadily increased over the past decade. According to the Leukemia & Lymphoma Society, a person in the US is diagnosed with leukemia, lymphoma, or myeloma every 3 minutes. In 2024 nearly 187,740 people in the US were diagnosed with these cancers. Allogeneic hematopoietic stem cell transplantation represents a potentially curative treatment approach for many of these high-risk and drug-resistant hematological malignancies.
Increased Research and Clinical Trials
Several research studies and clinical trials are underway to evaluate the safety and efficacy of allogeneic stem cell therapies for newer applications beyond traditional blood cancers and disorders. Several phase 2 and 3 trials are ongoing to assess allogeneic stem cell therapies for indications such as bone marrow fibrosis, osteoarthritis, stroke, traumatic brain injury, and myocarditis, among others. Robust pipeline activities focused on broadening the treatment landscape will provide new avenues for market growth. For instance, Novartis, a global pharmaceutical company, initiated a study using allogeneic stem cell transplantation for the treatment of bone marrow fibrosis.
Mergers and Acquisitions
The allogeneic stem cell therapy domain has witnessed significant merger and acquisition activity aimed at consolidating market presence as well as expanding cell therapy pipelines. For instance, in July 2022, Vertex Pharmaceuticals, a global pharmaceutical company, announced that it had entered into a definitive agreement to acquire ViaCyte, a biotechnology company focused on delivering novel stem cell-derived cell replacement therapies. Vertex Pharmaceuticals will accelerate ViaCyte’s VX-880 clinical trial program. VX-880 is an investigational allogeneic stem cell-derived, fully differentiated, insulin-producing islet cell therapy for T1D, which has already achieved proof-of-concept with highly promising safety and efficacy results from a phase 1/2 study.
Allogeneic Stem Cell Transplantation Market Opportunities- Favorable Government Regulations
Regulatory bodies like the U.S. FDA (Food & Drug Administration) and EMA (European Medicines Agency) have enacted expedited approval pathways, such as breakthrough therapy, fast track, and priority review, to accelerate approval timelines for promising regenerative medicine therapies. The U.S. FDA has also implemented the Regenerative Medicine Advanced Therapy designation to provide benefits to cell therapies showing significant efficacy in early studies. Such regulatory support and initiatives are creating a favorable environment for the growth of the allogeneic stem cell transplantation market.
Allogeneic Stem Cell Transplantation Market Challenges- High Cost of Cell Therapies
Allogeneic stem cell transplantation involves substantial upfront costs for specialized infrastructure, stringent manufacturing protocols, robust quality control, and skilled personnel. Complex handling and transportation requirements also add logistics expenses. Lack of adequate reimbursement and limited insurance coverage in several regions negatively impact affordability. The resultant high costs of therapy are a key restraint to the market growth.
Market Report Scope
Allogeneic Stem Cell Transplantation Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 4.53 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.8% | 2032 Value Projection: | USD 7.66 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Celgene Corporation, Mesoblast Ltd., Pluristem Therapeutics Inc., MEDIPOST Co., Ltd., Osiris Therapeutics, Inc., Cynata Therapeutics Limited, Takeda Pharmaceutical Company Limited, Astellas Pharma Inc., Cellular Biomedicine Group, Inc., Biosolution Co., Ltd., StemCell Technologies Inc., Caladrius Biosciences, Inc., ViaCord, Vericel Corporation, Regen Biopharma Inc., Gamida Cell Ltd., Histogenics Corporation, BrainStorm Cell Therapeutics Inc., and Lineage Cell Therapeutics, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Allogeneic Stem Cell Transplantation Market Key Developments
- In February 2025, Lineage Cell Therapeutics, Inc. announced the launch of the DOSED clinical study, focused on delivering oligodendrocyte progenitor cells (OPCs) for spinal cord injury using OPC1 device.
- In January 2025, Sana Biotechnology, Inc. announced positive initial results from a first-in-human study transplanting UP421, an allogeneic primary islet cell therapy engineered with its hypoimmune (HIP) technology, into a type 1 diabetes patient without immunosuppression. The study was conducted in collaboration with Uppsala University Hospital.
- In July 2024, the Transplantation and Cellular Therapy Program at Grand River Regional Cancer Centre (GRRCC) successfully carried out its first autologous stem cell transplant.
- On September 8, 2023, MaaT Pharma, a France-based biotechnology company, announced that the European Medicines Agency (EMA) has granted orphan drug designation to MaaT033, an adjunctive and maintenance therapy developed by MaaT Pharma to improve overall survival in allo-HSCT (Allogeneic Hematopoietic Stem Cell Transplant)
- In May 2023, Astellas Pharma, a global pharmaceutical company, and the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) announced top line results from the Phase 3 MORPHO clinical trial evaluating gilteritinib as a maintenance therapy following allogeneic hematopoietic stem cell transplantation (HSCT) for patients with FMS-like tyrosine kinase 3 (FLT3)-internal tandem duplication (ITD) mutated acute myeloid leukemia (AML)
Definition: Allogeneic stem cell transplantation involves transferring healthy stem cells from a genetically similar donor to the patient to replace damaged cells. It is an established therapeutic technique to treat various hematological cancers, blood, and immunological disorders. Allogeneic transplants can use stem cells derived from peripheral blood, bone marrow, umbilical cord blood, or adipose tissue. The field has seen significant advances in harvesting techniques, expansion protocols, cryopreservation, and cell processing. Key drivers are the rising incidence of blood cancers and autoimmune diseases, along with an increase in clinical trials assessing allogeneic stem cell therapies for various regenerative medicine applications.
Analyst Viewpoint
- The Allogeneic Stem Cell Transplantation Market is expected to witness substantial growth in the coming years. This growth is primarily driven by the increasing prevalence of hematologic malignancies and other non-malignant disorders, where allogeneic stem cell transplantation has proven to be a promising treatment option. Moreover, the growing investments in research and development activities, coupled with a robust pipeline of stem cell therapies, are expected to provide a significant push to the market. The increasing number of clinical trials focusing on the application of allogeneic stem cell transplantation in various therapeutic areas is a positive sign for the market's future growth. However, the high cost of transplantation procedures, lack of awareness, and stringent regulatory scenarios could pose challenges to the market growth.
- Regionally, North America is expected to dominate the market due to the advanced healthcare infrastructure, high prevalence of hematologic disorders, and presence of key market players. However, the Asia Pacific region is expected to exhibit the fastest growth rate owing to the improving healthcare infrastructure and increasing patient population. In conclusion, the Allogeneic Stem Cell Transplantation Market is poised for significant growth, driven by the increasing prevalence of diseases, advancements in technology, and a robust pipeline of therapies. However, factors such as high costs and regulatory challenges could potentially hinder the market growth.
Market Segmentation:
- By Source
- Bone Marrow
- Peripheral Blood
- Umbilical Cord Blood
- Adipose Tissue
- Dental Pulp
- Others
- By Cell Type
- Allogeneic MSCs (Allogenic Mesenchymal stem cells)
- Allogeneic HSCs (Allogeneic hematopoietic stem cells)
- Others
- By Application
- Cancer
- Autoimmune Diseases
- Blood Disorders
- Cardiac Disorders
- Neurological Disorders
- Diabetes
- Others
- By End User
- Hospitals
- Research Institutes
- Specialty Clinics
- Others
- By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
- Top Companies in the Allogeneic Stem Cell Transplantation Market:
-
- Celgene Corporation
- Mesoblast Ltd.
- Pluristem Therapeutics Inc.
- MEDIPOST Co., Ltd.
- Osiris Therapeutics, Inc.
- Cynata Therapeutics Limited
- Takeda Pharmaceutical Company Limited
- Astellas Pharma Inc.
- Cellular Biomedicine Group, Inc.
- Biosolution Co., Ltd.
- StemCell Technologies Inc.
- Caladrius Biosciences, Inc.
- TiGenix N.V.
- ViaCord
- Vericel Corporation
- Regen Biopharma Inc.
- Gamida Cell Ltd.
- Histogenics Corporation
- BrainStorm Cell Therapeutics Inc.
- Lineage Cell Therapeutics, Inc.
Sources
Primary Research Interviews with Following Stakeholders:
- Healthcare providers (hospitals, specialty clinics), stem cell therapy companies, regulatory authorities, and research institutions, interviews with medical professionals, hospital directors, and researchers.
Databases
- WHO Global Observatory on Donation and Transplantation
- GlobalData (for clinical trials and market trends)
- National Marrow Donor Program (Be The Match) – data insights
- ClinicalTrials.gov
- World Bank and OECD health databases
Industry Magazines
- BioPharma Dive
- GEN (Genetic Engineering & Biotechnology News)
- PharmaVoice
- MedTech Insight
Academic and Trade Journals
- Biology of Blood and Marrow Transplantation (BBMT)
- Bone Marrow Transplantation (Nature)
- Journal of Hematology & Oncology
- The Lancet Haematology
- Transplantation and Cellular Therapy
Reputed Newspapers
- The New York Times – Health Section
- The Guardian – Science and Health
- The Times of India – Health and Research
- The Wall Street Journal – Pharma & Healthcare
Industry and Medical Associations
- World Marrow Donor Association (WMDA)
- American Society for Transplantation and Cellular Therapy (ASTCT)
- European Society for Blood and Marrow Transplantation (EBMT)
- Asia-Pacific Blood and Marrow Transplantation Group (APBMT)
- International Society for Cellular Therapy (ISCT)
Public Domain Sources
- WHO Reports
- U.S. FDA and EMA (European Medicines Agency) regulatory updates
- National Institutes of Health (NIH)
- Health ministry reports and national health registries
- Company annual reports, investor presentations, and press releases
- Global Burden of Disease (GBD) database
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients