Key milestones of COVID-19 Pandemic
- 31st December 2019 – First case of coronavirus was identified in Wuhan, China
- 12th January 2020 – China makes genome sequencing of novel coronavirus publicly available
- 13th January 2020 - First case of novel coronavirus outside of China confirmed
- 30th January 2020 - Outbreak was declared a Public Health Emergency of International Concern
- 11th February 2020 - WHO announced a name for the new coronavirus disease. COVID-19
- 12th February 2020 - Research and innovation forum sets priorities for COVID-19 research; UN activates WHO-led Crisis Management Team
- 22nd February 2020 – 11,000 African health care workers were trained on COVID-19
- 1st March 2020 - UN releases US$15 million for COVID-19 response
- 3rd March 2020 - WHO shipped nearly half a million sets of personal protective equipment to 47 countries, but the global supply is rapidly depleting
- 7th March 2020 – Number of COVID-19 cases reached 100,000
- 11th March 2020 – WHO characterized COVID-19 as a pandemic
- 13th March 2020 - WHO, UN Foundation and partners launch first-of-its-kind COVID-19 Solidarity Response Fund; President Trump declares a national emergency.
- 16th March 2020 - Latin America begins to feel the effects of the novel coronavirus
- 17th March 2020 - The E.U. bars most travelers from outside the bloc for 30 days
- 19th March 2020 - For the first time, China reports zero local infections
- 23rd March 2020 - Prime Minister Boris Johnson announces lockdown in Britain
- 24th march 2020 – Indian announced lockdown of 21 days; Tokyo Olympica delayed untill 2021
About the pandemic
Pandemics of infectious diseases have always created a havoc globally. While pharma giants have been busy researching treatments for cancer, rare diseases and neurological disease by large, every consultant across the world is wondering, whether the treatment for infectious disease been eliminated from their priority list?
Amid the outbreak of Nipah virus, swine flu, ebola and now corona virus, the question to ponder is that Why the global pharmaceutical fraternity, including the World Health organization (WHO), is not prioritizing the discovery of prevention/treatment solutions for such outbreaks.
Novel coronavirus, also referred as COVID-19 (Coronavirus detected in 2019), was first detected in Wuhan, China on 31st December 2019. Since then, in a span of 70 days, it affected more than 100,000 people globally. According to the WHO data, there were 9,800 cases identified in January 2020, which spiked to 85,600 cases by the end of February 2020. A staggering 770% increase.
Coronaviruses (CoV) are a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). Coronaviruses are zoonotic, meaning they are transmitted between animals and people. A novel coronavirus (nCoV) is a new strain that has not been previously identified in humans.
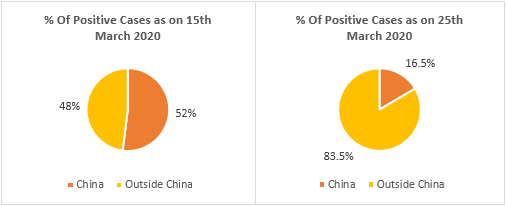
A total number of 413,467 cases were reported by WHO as of 25th March 2020. Major affected regions/countries/territories include China (81,848 cases), Italy (69,176 cases), Iran (24,811 cases), Republic of Korea (9,137 cases), Spain (39,673 cases), Germany (31,554 cases), and France (22,025 cases) – as of 25th March 2020. China accounts for more than 50% of the cases globally. This has reduced significantly in recent days owing to high prevention and control measures taken by the Chinese government. Coherent Market Insights estimates the corona infected population to reach 1,950,000 by April 2020. Globally 26,654 deaths were reported by WHO as of 25th March 2020. Of which, 6,820 deaths (25%) were reported from Italy.
Every day, a new country/territory/region reports a case of COVID-19. According to the statistics, all first cases identified in countries other than China are imported cases only. A delay in diagnosis, leads to the massive spread of the infection.
Impact on economy
The fear of COVID-19 is clearly visible on the stock markets globally. A daily breakdown of 4%-7% has led the stock markets break all its long term support levels and reach a new low. This definitely is an opportunity for long term investors to buy stocks at a very lower price. However, it is necessary to understand the impact on the global economy at a much broader level.
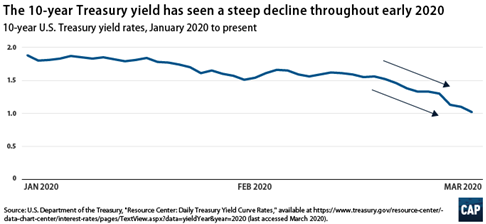
- The bond yields have come down drastically
- Brent crude which was trading at US$ 57.67 on February 17, 2020, touched US$ 25.6 on March 25, 2020
- China is the major exporter of active pharmaceutical ingredients. A complete lockdown will impact the global pharmaceutical market in the coming few months.
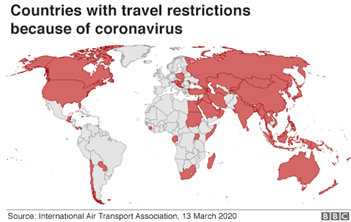
- Countries have suspended visas and tourists amidst the fear of COVID-19 spread in the unaffected or less affected regions. This has and will significantly impact the travel industry, mainly the airlines. Already bleeding airline sector, is expected to near the bankruptcy or at least see a dramatic rise in debt in coming few months owing to this.
- The U.S. Federal Reserve reduced its key interest rate to zero on 11th March 2020, a move seen for the first time after 2008 crisis
- The auto industry is also thought to be impacted greatly, as China accounts for a lot of production for the U.S. companies.
- iPhone, and Diet Coke are some of the key products to be impacted owing to this pandemic. Apple closed its stores in China on February 2, 2020.
- The global supply chain has been impacted due to COVID-19 infection. This has impacted every industry globally.
- Sport events and matches have been cancelled on the fear of COVID-19
- Hotels, restaurants, clubs, malls and movie theatres have experienced a dramatic fall in bookings and customer footfalls amidst the COVID-19 pandemic
- The price of gold - which is often considered a "safe haven" in times of uncertainty - has also increased. It was trading at a 7-year high in February.
At the same time, there are some unaffected/positively affected sectors as well:
- Demand for wipes, sanitizers, and disposable medical supplies have increased to an extent that the supply is unable to meet the demand

- Cost of wipes, sanitizers, and disposable medical supplies have increased more than 250%
- Agriculture is the sector expected to be least impacted amidst coronavirus pandemic, mainly because no chances of crowd gathering
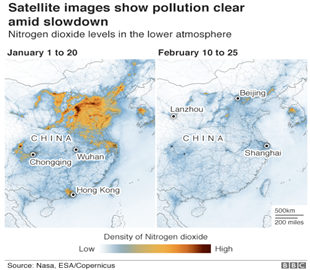
- The NASA image depicts decrease in pollution on account of lockdown in various regions
Diagnostics
On March 13, 2020, the U.S. FDA provided an emergency clearance for two diagnostic tests – one from Roche and one from Thermo Fisher. The tests were approved within 24 hrs. of receiving the application from respective companies. Roche diagnostic kit is intended to be used with its cobas 6800/8800 molecular testing systems. Roche has the capacity to manufacture 400,000 tests (units) a week. On the other hand, the diagnostic test from Thermo Fisher is intended to be sued with its Applied Biosystems 7500 Fast Dx Real-time PCR instrument. Thermo Fisher plans to produce 5 million tests in a month.
A laboratory-based COVID-19 test takes around 5 hours to deliver results. U.K.-based Mologic Ltd., in collaboration with Senegalese research foundation Institut Pasteur de Dakar, developed a handy testing kit that can test the COVID-19 in just 10 minutes. This test, expected to be ready for sale by June 2020, for less than a US$ 1, is a transformational product in this emergency.
Treatment approaches
Pharmaceutical companies have taken immediate action in terms of identifying and developing drugs for treating COVID-19. In addition, companies are also researching for vaccines against this novel coronavirus. More than 35 treatments (including drugs and vaccines) are in development for coronavirus. HIV drugs are experimented on a huge scale as a potential treatment option for COVID-19 virus. This is only expected to increase as global health agencies, governments, and drug companies collaborate to put a common efforts against the SARS-CoV-2/COVID-19 virus.
Serum Institute of India plans to introduce COVID-19 vaccine by 2022. It has already initiated pre-clinical trials of the vaccine candidate. GlaxoSmithKline, J&J, Moderna Inc., and Sanofi are also working on to develop a vaccine for this virus. In all, there are more than 12 companies working towards developing a vaccine or drugs for coronavirus treatment or prevention.
However, the immediate potential treatment options include Remdesivir (Gilead) and Actemra (Roche). Roche has donated US$ 2 mn worth of Actemra to China based on its usage recommendation from China’s National Health Commission. Cost of Actemra is US$ 491 for a supply of 4ml IV solution. Both these drugs are being studies in phase 2 and 3 trials for coronavirus.
The mortality rate in China has decreased to 3.9% (as on 15th March 2020) which was once around 5% at the peak of coronavirus infection in China.
Current drugs approved for treatment:
- Favilavir (formerly known as Fapilavir)
- Chloroquine Phosphate
- Remdesivir
- Tocilizumab (Actemra)
Potential drugs under research and development:
- Leronlimab
- Lopinavir/ritonavir
- Rintatolimod
- APN01
- Danoprevir
- ASC09
- IFX-1
- Galidesivir
- BXT-25
- CYNK-001
- Camrelizumab and thymosin
- Brilacidin
- Darunavir and Cobicistat
- Umifenovir
Market Potential
With the possible assumptions of Actemra to be available at a discounted price in such pandemic situations, we estimate the market potential for the approved treatment to be as below:
- Patient population (as on 25th march 2020): 413,467
- Assumed Treatment Cost: US$ 200
- Estimated Monthly Market Size: US$ 82 mn
- Forecasted patient population in April 2020: 1,950,000
- Assumed Treatment Cost: US$ 200
- Estimated Monthly Market Size: US$ 390 mn
With estimated monthly revenue of almost US$ 100 mn, corona treatment would account for more than 40% of Actemra’s current revenue (US$ 2.4 bn).
A vaccine, on the other hand is estimated to have a potential market of more than US$ 10.0 bn in China itself (considering the cost of the vaccine to be US$ 10)
So the question now is, if infectious diseases pose such a huge potential, why the pharmaceutical industry does not still pursue to invest in R&D for such pandemics?
Initiatives and Funding
The World Health Organization (WHO) is providing regular updates, and guidelines about coronavirus to create awareness. Also, global health agencies, and governments are creating an emergency fund for combatting coronavirus.
The U.S. government, approved US$ 8.3 billion emergency fund to treat and prevent the spread of novel coronavirus, COVID-19. This fund will be utilized for R&D, procurement of vaccines, therapeutics, diagnostics, support the state and local health agencies in the U.S., and spreading awareness on international level.
On 13th March 2020, the WHO, UN Foundation and partners launched first-of-its-kind COVID-19 Solidarity Response Fund. Individuals, organizations and governments are welcomed to donate amount in this fund that would support the action from WHO’s COVID-19 Strategic Preparedness and Response Plan.
On 13th March 2020, the European Commission proposed to direct an amount of EUR 37 billion to fight against the Coronavirus crisis.
Iran, which is among the affected countries in this pandemic, has asked for a US$ 5 billion emergency fund from the International Monetary Fund (IMF) to fight novel coronavirus in its country. Iran has been economically impacted since a long time, amidst US government sanction on oil and gas exports. Also, with countries closing their borders, the economy is further impacted owing to no oil and gas export.
- WHO continues to advice against the application of travel or trade restrictions to countries experiencing COVID-19 outbreaks
- It developed a COVID-19 Strategic Preparedness and Response Plan to combat the pandemic universally
- Quest Diagnostics and LabCorp have rolled out a new COVID-19 coronavirus test service
- Indian government and also the government of other countries have asked insurance companies to include coronavirus diagnostic testing cost under their plans
- Under public services, coronavirus testing is done free of cost in many countries
Fig. 1: Number of additional health measures by type, WHO, 10th March 2020
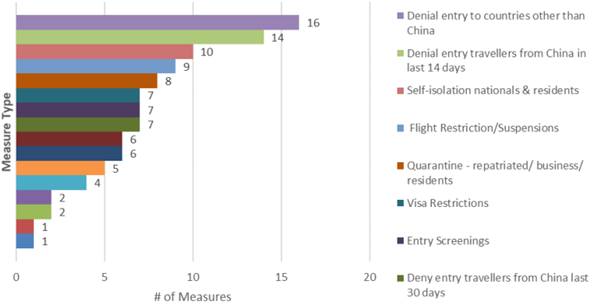
A number of initiatives are expected to arise in the coming days, as the situation only worsens in regions outside China.
The world has been inadequately equipped to tackle epidemics and pandemics. Investments in defense are rising, however preparation for fighting infectious diseases is inadequate. Real danger to the human race are these invisible organisms and not the visible lives. Human’s strengths have been tested a many times recently with epidemics/pandemics such as Swine Flu, SARS, Ebola, and Nipah virus. The positive sunshine, after the dark of the dawn is that, the world has united to fight against this pandemic and prevent further deaths.
In this unprecedented time, the COVID-19 pandemic is having an impact on the health of our loved ones, the businesses we rely upon, the health of the global economy, and the way we live our daily lives. As every business continue to navigate through these unique and evolving challenges, humanity is hopeful that together it will overcome it, by bringing a cure (drug and vaccines) in the form of the silver living to the surrounded dark clouds of uncertainty, and terror.
Data Sources: WHO, BBC, UN News, Bloomberg, Center for American Progress, Roche Annual Report, Press Releases, News Websites, Coherent Market Insights
Author –
Saurabh Shah,
Principle Consultant – Healthcare,
Coherent Market Insights
About Coherent Market Insights
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions. We are headquartered in India, having office at global financial capital in the U.S. Our client base includes start-ups, not-for profit organization, and fortune 1,000 companies across various business verticals in over 49 countries worldwide. We are uniquely positioned to help businesses around the globe deliver practical and lasting recommendations by providing holistic insights on operational improvements, technologies, emerging market trends and revenue impacting decisions. We offer both customized and syndicated market research reports that help our clients create visionary growth plans for their businesses. Our global team of over 100 research analysts and contract consultants meticulously study emerging trends across various industries at both the global and regional levels to identify new opportunities for our clientele. For more information, please visit: https://www.coherentmarketinsights.com/






